Antioxidant Mitochrondria Biogenesis Supplements
I will be stocking up on the exercise mimetic supplements shortly. I decided to purchase these from www.Purebulk.com as they have the full range I require.
Initially I was going to buy green tea extract for the polyphenols and PQQ but I already drink plenty of green and oolong tea – which incedentally increases mitochondria when consumed with vitamin B. However as green tea extract and the ployphenols are highly liver toxic I willl omit this supplement and just continue to drink the tea along with vitamin B.
Here is the list of what I will purchase and it should come to a years supply at least.
Coenzyme Q10 (COQ10) 2x 25 gm – a one year supply – cost $45 – must be melted into coconut oil to avoid spoilage.
Grape Seed/Pine Bark Gel-Caps 600 x 450mg – $54
Grape seed and pine bark extracts act as antioxidants and promote a healthy cardiovascular system. Antioxidants are helpful because they fight and destroy free radical molecules, which can damage cell membranes.
Grape seed extract also contains resveratrol. This chemical is found primarily in the skin and seeds of grapes. Resveratrol is also an antioxidant, providing support for the health of the cardiovascular system.
Grapefruit Seed Extract 250g – cost $24
Refer the the Bill Brinks article in a previous post for information on grapefruit extracts – basically it contains mitochondria biogenesis nutrients
Quercetin Dihydrate P.E. 95% 500g – $100
In total $224 plus shipping for over a years supply. Shipping to New Zealand can run a little steep but it will still be under $300nz I hope – in return I get the mitochondria levels of a fit young man.
High Mitochondria equals high aerobic capacity and Mitochondria also BURN FAT !!!
One last supplement I will add to the stack is Acetyl-L-Carnitine
Produced by the body in the liver and kidneys, L-Carnitine is a nutrient that helps the body turn fat into energy. By transporting fatty acids into the mitochondria, L-Carnitine boosts energy levels, because it stimulates the body’s burning of triglycerides as fuel. These characteristics result in burning fat, increasing energy and improving resistance to muscle fatigue. Not surprisingly, these properties make L-Carnitine a popular supplement with the bodybuilding community – and an ideal way to initiate fatty liver disease regression.
In a double-blind study, Dr. Malaguarnera and colleagues randomized 74 patients with NASH to receive either L-Carnitine (a 1 gram tablet after breakfast and another after dinner) or placebo for 24 weeks. All participants were put on a 1,600-calorie/day diet and a whole-body stretching routine performed three times a week. At the end of the study, those taking the L-Carnitine supplements had considerable improvements in their liver function, glucose plasma level, lipid profile and liver histology. The authors concluded that L-Carnitine and lifestyle changes, including weight loss and exercise, represent viable therapeutic options in NASH.
Lower Core Temperature for 30% Performance Gain
There are currently some good solutions to lower core body temperature while you work out. This can have quite phenomenal performance benefits. The expensive option is CoreControl a special cooling glove developed by Stanford scientists.
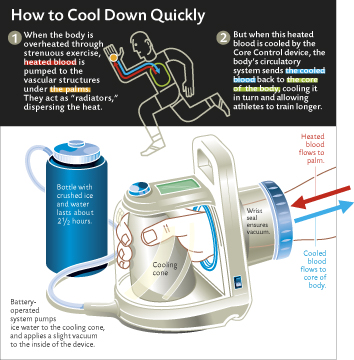
http://www.stanfordalumni.org/news/magazine/2005/julaug/features/cool.html
http://www.avacore.com
These things cost $3000 so for me its not an option. What is an option is a vest, cap and bexrunner palm cooler. It may sound dumb that cooling your hand will affect core body temperature but it does. One athlete went from 200 to over six hundred pullups per workout in a six week period using the expensive cooling glove.



Your face, palms and soles of the feet are the main places that lose body heat. The palms have a strong neurological connection and also regulate body temperature. There are specialised thermo-regulation blood vessels at these body parts.
Heres an article on the CoreControl Glove
BY Eva Ciabattoni
http://www.stanfordalumni.org/news/magazine/2005/julaug/features/cool.html
Robert Weir, head coach of men’s track and field, gets ready for his strength-training regime by loading hundreds of pounds of weights onto both ends of a bar that rests in brackets at shoulder height. Weir moves under the bar, hoists it across his shoulders and does squats. With each repetition, his knees and hips fold until his thighs are parallel to the ground, then straighten—rep after rep with the equivalent of a baby elephant draped around his shoulders.
Like any athlete, Weir is well acquainted with his normal performance range. Like any athlete, Weir looks for an edge. A few years ago, he was intrigued when he heard about a device—that has been called at various times the RTX, Core Control or simply The Glove—invented by a pair of Stanford biologists. Using the device to lower his core body temperature between sets, he was able to lift 495 pounds in four sets of squats instead of his normal two. He usually does squats only on Mondays, but he decided to try a second series a few days later. That Friday, he was able to increase the weight to 545 pounds. “I was surprised the sets felt so good,” he says, but adds that the real test came the following Monday. Weir, 44, expected to see significant performance degradation due to the extra Friday workout. Not only did he not see the decay, he increased weight with every set. The RTX—for rapid thermal exchange—cooling device “is a very serious piece of equipment,” he says. “At my age, you don’t expect to be setting personal bests during workouts.” He trained with the cooling equipment for the 2002 Commonwealth Games, and placed third in the discus. His oldest competitor was 15 years younger.
RTX promises to enhance human performance in applications ranging from sports to medicine to the military. It is the brainchild of biological sciences professor H. Craig Heller and senior research scientist Dennis Grahn, who have spent nearly two decades studying temperature regulation in mammals. Their lab, once devoted to hibernating ground squirrels and marmots, now attracts San Francisco 49er football players, military representatives from the Defense Advanced Research Projects Agency, multiple sclerosis patients and sweating Stanford athletes.
COLD SHOULDER: Grahn and Heller got a chilly reception from some scientists when they first published their findings in 1998.
The fourth floor of Gilbert Biological Sciences Building holds many iterations of Heller and Grahn’s inventions to heat and cool humans. One of the earliest contraptions circulated warm water around an arm encased in a clothes-dryer duct and sealed with neoprene. It was designed to warm patients recovering from anesthesia.

The drugs that render patients unconscious also make them hypothermic. That’s useful because chilled patients bleed less during surgery; but as they start to wake their violent shivering can tear fresh sutures, damage teeth and put extra stress on heart and lungs. It’s important to warm patients quickly after a procedure, and traditionally that’s been done with hot air or blankets. The trouble, Heller explains, is that because the body’s core is still cold, blood flow is pulled away from the skin to preserve internal body heat. Warm blankets heat the skin, but without a ready supply of blood circulating near the skin surface, this warmth is not transferred efficiently to the body’s interior.
Heller and Grahn found that heating only an arm with their device served to warm patients more rapidly than would have been expected via normal heat transfer through the percentage of skin surface being heated. After modifying their design, they realized that they could achieve the same rate of rewarming by encasing just the hand.
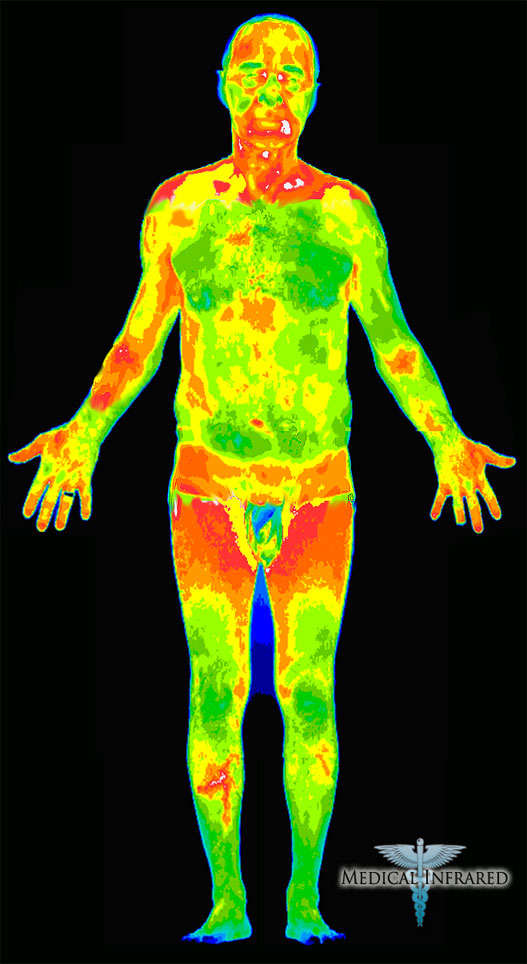
Mammals have specialized blood vessels in their palms and other hairless skin surfaces—ears, nose, cheeks and soles of the feet—that are designed to dissipate heat. (These radiator-like structures—venous plexuses and arteriovenous anastomoses—were described as early as 1858 in Gray’s Anatomy.) By redirecting blood away from the capillaries and into these blood vessels, the body can shed heat quickly. What Heller and Grahn were seeing was the return trip: when externally applied heat shocked open the radiators in the cold palms of anesthesia patients, warmed blood was returned straight to the heart, and the body was reheated from the inside out. Applying a mild vacuum to the hand intensified this effect.
Their finding that heat loss is not uniform across the body was slow to gain acceptance. In the Journal of Applied Physiology, where their research was first published in 1998, Heller and Grahn issued a frosty rejoinder to skeptics: “since we present not just a claim but hard data, it is nice to emphasize that when data do not fit a model it is time to reexamine the model.”
Nigel Holmes
After hearing of their rewarming research, a postdoctoral student in molecular biology and neuroscience approached them to see if the same radiator mechanism could be used to cool the body core. Louise Bitting, PhD ’94, had read that the cooling of cells containing the cystic fibrosis mutation had halted the disease process in those cells. She wanted to study if cooling might work outside a Petri dish. (Bitting died unexpectedly in 1999 at the age of 49.)
A lab technician who was also a body builder, Vinh Cao, volunteered to be the test subject. To generate metabolic body heat, Heller and Grahn had him do sets of pull-ups to exhaustion. He started with a set of 14 pull-ups and soon dropped to eight per set. After 20 minutes, they applied cooling and a vacuum to Cao’s hand. When they asked him to do more pull-ups, they were amazed to see his performance jump back up to 14 pull-ups. To make sure the improvement wasn’t caused by the rest period, they did a study without cooling. Cao did 10 pull-ups.
They continued to study Cao for the next six weeks. If they applied cooling between sets, Cao’s performance held steady in set after set. Without cooling, it decayed. “It was as if he had no fatigue,” Heller recalls. “We saw incredible gains over the next six weeks. He tripled his capacity to 620 pull-ups.” Preventing muscle exhaustion allowed Cao to train harder, leading to rapid gains in muscle strength. Heller and Grahn theorize that more blood, and thus, oxygen, is available to the muscles when the body doesn’t have to route extra blood to the radiators for cooling.
Excited by what they had learned, they arranged a presentation in 2000 to Stanford athletics coaches. Heller remembers the stony faces and crossed arms that greeted them. “It was not a warm welcome. The four or five coaches who showed up didn’t seem to think that a couple of biologists could tell them anything about performance enhancement.” Only Weir agreed to try it. Off campus, the 49ers and Raiders football teams were the earliest adopters—later followed by the University of Miami football team, the NBA’s Milwaukee Bucks and the Manchester United soccer team.
When a battery-operated model of the RTX became available, the Stanford football team started to use it. Head athletic trainer Charlie Miller made an inadvertent breakthrough when one his players came off the field with leg cramps during the third quarter of a game against Boston College early in the ’02-’03 season. “Since cramps tend to recur, a coach has to decide between benching a key player or keeping him on the field and risk another cramp recurring in the middle of play,” Miller explains. In addition to conventional treatments—massage, electrolytes, fluids—Miller had him put his hand into the RTX. To his surprise, the cramp disappeared and the player was able to finish the game. “When the IV fluids worked [to revitalize a player], it wasn’t the minerals or the rehydrating,” he says, “it was because we were invasively cooling the players down. We had noticed that if the IVs were kept on ice, they worked better. Now we know why.”
Miller says the RTX is a competitive advantage because it allows a coach to keep his best players on the field. Because the device is so new, there are no requirements yet for the host team to provide one to the visiting team, as there are for other amenities.
Ever seeking a competitive edge, athletes began paying regular visits to the fourth floor of Gilbert, causing one of the staff to remark that the hallways had gotten smaller. A former NFL player told Grahn, “This replaces the Juice,” referring to steroids. Weirdly, cooling does mimic steroids in the way it allows an athlete to recover from intense exertion quickly, allowing someone to do more work in a shorter period of time. But cooling doesn’t result in shriveled gonads or ’roid rage.
Cooling mimics steroids in the way it allows an athlete to recover from intense exertion quickly. But cooling doesn’t result in shriveled gonads or ’roid rage.
Critics might worry that cooling masks the body’s signals to stop. In fact, lab data show that athletes who train with cooling perform better in all kinds of conditions—even competitions when cooling is un-available. Heller says removing heat from the body is no different from giving it a drink of water in response to thirst. Asked whether training with cooling might lead to overuse injuries, Weir shakes his head. “It doesn’t allow you to do work you couldn’t ordinarily do. It allows you to recover faster.”
Meanwhile, researchers continue to investigate therapeutic uses for cooling. One exciting area of research involves multiple sclerosis, a disease where even a 1/2-degree Celsius rise in core body temperature can lead to rapid and dramatic physical and cognitive decline. (MS sufferers say the sudden enervation feels as though a switch was flipped.) The disease destroys portions of the fatty myelin sheath that insulates nerves; heat disrupts the electric impulses traveling along the frayed nerves. Retaining strength—key to staying out of a wheelchair—is a significant challenge for MS patients, for whom fatigue can lead to a spiral of debility.
Jim Seaton, a management consultant who lives in Washington, D.C., has MS. Once a top runner and avid hiker, he has to be cautious about exertion. He pushed himself too far once and had to crawl back to his car in the parking lot; recovery to his baseline level of functioning took two days. Seaton, after hearing a radio report about cooling athletes, arranged to try the RTX to see if it reduced the fatigue that resulted when his body warmed up. Using the RTX, he can cool to his resting state in 10 to 15 minutes—and then continue to hike. The RTX isn’t exactly convenient: the $4,000 unit weighs 12 pounds and has to be reloaded with ice every 2 1/2 hours. But owning one changed his life. “I’m already planning trips to the museum [and] to Europe that I would have thought thrice about before.”
At their boneyard of core-cooling machines in the Gilbert building, Heller and Grahn describe the difficulty in perfecting the design for a functional, portable RTX. There’s the coffeepot-shaped version. The $400,000 version by a name-brand design firm that really never worked. The version constructed in a size-10 boot that, once loaded with tubes and a cooling surface, wouldn’t fit on even a size-5 foot. Grahn’s latest homemade version features soft vinyl against the hand instead of metal. One design challenge is obvious—how to create a vacuum-bearing glove flexible enough so that its wearers can use their hands, not just sit cooling their palms.
Variable temperature control is another desirable feature. When a hot body core issues a command to open the radiators and dump heat, the palm can override that command and order the radiators to shut down based on local conditions, i.e., if the palm touches a cold surface. This was borne out in February when Grahn flew to Alaska to observe dog teams competing in the Iditarod. Temperatures rose to 46 degrees in Anchorage—downright tropical for the huskies. Grahn watched sled dogs through an infrared camera—and saw snouts and ears lit up like headlamps, indicating that the dogs were shedding excess body heat. But the cameras showed no heat loss through the dogs’ feet. Snow under their paws prevented those radiators from opening. Heller and Grahn have found in the lab that the temperature under which the radiators shut down in humans is highly individual.
Heller and Grahn have received a series of patents through Stanford’s Office of Technology Licensing, which will share in any royalties. They are founders and major stakeholders in AVAcore Technologies, a Michigan firm charged with making the RTX commercially viable. (The company moved from the Bay Area to Ann Arbor in 2003 to take advantage of engineers laid off from the automotive industry.) “It’s hard to build a compressor small enough to be useful in portable situations,” says Ronald Piasecki, chief executive officer of AVAcore. “Eventually nanotech may play a role in accomplishing our engineering goals.”
Piasecki has overseen improvements to the RTX manufacturing process, reducing the cost and time to build each Core Control machine. “Clearly, the athletic market is the low-hanging fruit,” he says of the 100 units sold so far. “But this fall we’re starting a study of MS patients in conjunction with the University of Michigan neurology department.”
Heller remains confident that the technology can be brought to wide markets. “There are many applications of both heating and cooling,” he says. “Firefighters, soldiers in full gear in the Iraq desert, stroke victims [where cooling patients can prevent further damage], cancer patients [where heating can increase effectiveness of chemotherapy drugs], cystic fibrosis, heatstroke victims”—all are potential beneficiaries of RTX.
Mighty Mitochondria Supplements
OK – This is exciting and easy to implement…
There are now several options for increasing Mitochondria count.
1. Episodic dietary fasting periods such as warrior diet.
2. Intense strength / endurance training that builds work capacity
3. Vitamin B / Green tea cocktails – search for the pepperdine study for more info
4. Quercitine, PQQ and other supplements
Here are some links and an article:
http://www.naturalmedicinejournal.com/article_content.asp?edition=1§ion=2&article=95
http://www.brinkzone.com/articles/exercise-mimetics-mitochondrial-boosters/
Editor’s Blog
Rejuvenate Your Cells by Growing New Mitochondria
By: Kirk Stokel
Published: March 21, 2011
Mitochondrial dysfunction is a primary cause of age-related decline. [1-7] In a revealing study, a team of researchers showed that muscle tissue of a 90-year-old man contained 95% damaged mitochondria compared to almost no damage in that of a 5-year-old. [8] When one looks at the boundless energy of a child compared to an elderly person, the devastating impact of mitochondrial degradation become instantly apparent. A myriad of recent scientific reports link defective and deficient mitochondria to virtually all degenerative diseases, including Alzheimer’s, type 2 diabetes, heart failure, and cancer. [9-13] Up until now, the best we could do was protect and improve the function of existing mitochondria using nutrients like L-carnitine, lipoic acid, and coenzyme Q10.
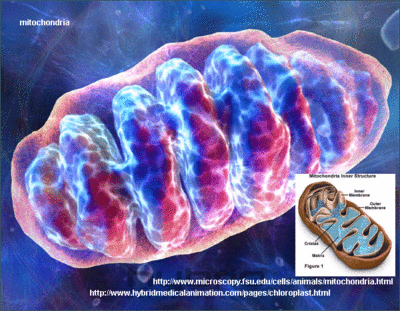
In an unprecedented breakthrough, a compound has been discovered that promotes the growth of new mitochondria structures within aging cells. [14] In this article, you will discover how this novel compound can help reverse cellular aging by activating genes that stimulate mitochondrial biogenesis, which means the generation of new mitochondria.
Mitochondria are the only cell components (other than the nucleus) to possess their own DNA. This means mitochondria have the ability to replicate and increase their number within a single human cell. Human cells may house anywhere from 2 to 2,500 mitochondria, [15-17] depending on tissue type, antioxidant status, and other factors. A growing number of biologists espouse the theory that mitochondrial number and function determine human longevity. [18-20]
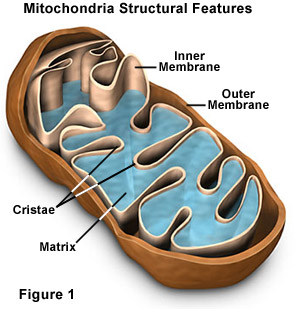
To put it simply, the more functional mitochondria you have in your cells, the greater your overall health and durability. The problem is that as we age, our mitochondria degrade and become dysfunctional. Age-related destruction of the mitochondria occurs more rapidly than in other cell components, meaning that for most people, it is loss of functional mitochondria that ultimately leads to personal extinction. The challenge aging humans face is that methods to increase the generation of new mitochondria are difficult to adhere to. Up until recently, the only natural ways to stimulate mitochondrial biogenesis were calorie restriction or exhaustive physical activity.
A natural agent with the power to safely induce mitochondrial biogenesis would mark an extraordinary advance in the quest to halt and reverse cellular aging. A compound called pyrroloquinoline quinone or PQQ is rapidly emerging as that nutrient.
PQQ: A Quantum Leap That May Reverse Cellular Aging
PQQ (pyrroloquinoline quinone) plays a critical role across a range of basic life functions. As an ultra potent antioxidant, it provides extraordinary defense against mitochondrial decay: PQQ’s chemical structure enables it to withstand exposure to oxidation up to 5,000 times greater than vitamin C. [21] When combined with CoQ10, research shows just 20 mg per day of PQQ can significantly preserve and enhance memory, attention, and cognition in aging humans. [22] But the most exciting revelation on PQQ emerged early in 2010, when researchers found it not only protected mitochondria from oxidative damage—it also stimulated growth of new mitochondria!
PQQ (pyrroloquinoline quinone) plays a critical role across a range of basic life functions. As an ultra potent antioxidant, it provides extraordinary defense against mitochondrial decay: PQQ’s chemical structure enables it to withstand exposure to oxidation up to 5,000 times greater than vitamin C. [21] When combined with CoQ10, research shows just 20 mg per day of PQQ can significantly preserve and enhance memory, attention, and cognition in aging humans. [22]
But the most exciting revelation on PQQ emerged early in 2010, when researchers found it not only protected mitochondria from oxidative damage—it also stimulated growth of new mitochondria! [14]
PQQ Is an Essential Micronutrient
PQQ is ubiquitous in the natural world. It has been found in all plant species tested and is present in human milk. Humans, however, are not capable of synthesizing it. [23] This has led researchers to classify PQQ as an essential micronutrient. PQQ’s potential to stimulate mitochondrial biogenesis was foreshadowed by early findings indicating its central role in growth and development across multiple forms of life.
PQQ has been shown to be a potent growth factor in plants, bacteria, and higher organisms. [21,24,25] Pre-clinical studies reveal that when deprived of dietary PQQ, animals exhibit stunted growth, compromised immunity, impaired reproductive capability, and most importantly, fewer mitochondria in their tissue. Rates of conception, the number of offspring, and survival rates in juvenile animals are also significantly reduced in the absence of PQQ. [26-28] When PQQ is introduced back into the diet, it reverses these effects, restoring systemic function while simultaneously increasing mitochondrial number and energy efficiency.
These compelling data prompted a team of researchers at the University of California- Davis to specifically analyze PQQ’s influence on cell signaling pathways involved in the formation of new mitochondria. [14] Their work, published last year, led to several extraordinary discoveries. They found that PQQ’s critical biological roles stem from its ability to activate genes directly involved in cellular energy metabolism, development, and function. [14]
Their findings shed light on results from favorable prior studies. For example, PQQ deficiency in juvenile mice results in a 20-30% reduction in the number of mitochondria in the liver, elevated blood glucose, and impairment in oxygen metabolism. [26] These are hallmark indicators of mitochondrial dysfunction. Yet when PQQ was put back into the diet, these pathological effects were reversed, along with an increase observed of new mitochondria. This and additional animal model data [28] taken together confirm PQQ’s ability to significantly boost mitochondrial number and function—a key to cellular anti-aging and longevity.

How PQQ Generates New Mitochondria
Mitochondrial biogenesis can be defined as the growth and division of pre-existing mitochondria. This phenomenon is not only accompanied by increased mitochondria numbers, but also their size and mass.
Mitochondrial biogenesis requires the coordinated synthesis and import of 1,000- 1,500 proteins where they facilitate the production of healthy new mitochondria.
Mitochondrial biogenesis occurs through the combined effects of genes activated by PQQ via the following three mechanisms:
PQQ increases expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha or PGC-1a. pGC-1a is a “master regulator” gene that mobilizes your cells’ response to various external triggers. It directly activates genes that boost mitochondrial and cellular respiration, growth, and reproduction. Its capacity to modulate cellular metabolism at the genetic level favorably affects blood pressure, cholesterol and triglyceride breakdown, and the onset of obesity. [29]
PQQ activates a signaling protein known as caMp-response element-binding protein or CReb. The CreB gene plays a pivotal role in embryonic development and growth. It also beneficially interacts with histones, molecular compounds shown to protect and repair cellular DNa. CreB also stimulates the growth of new mitochondria. [30]
PQQ regulates a recently discovered gene called dj-1. as with pGC-1a and CreB, DJ-1 is intrinsically involved in cell function and survival. It has been shown to prevent cell death by combating intensive antioxidant stress and is of particular importance to brain health and function. DJ-1 damage and mutation have been conclusively linked to the onset of Parkinson’s disease and other neurological disorders. [31-34]
Protecting Against Mitochondria- generated Free Radicals
As the primary energy engines of our cells, the mitochondria rank among the structures most vulnerable to destruction from oxidative damage. The formidable free radical-scavenging capacity of PPQ furnishes the mitochondria considerable antioxidant protection. At the core of this capacity is an extraordinary molecular stability. [35] As a bioactive coenzyme, PQQ actively participates in the energy transfer within the mitochondria that supplies the body with most of its bioenergy (like CoQ10).
Unlike other antioxidant compounds, the stability of PQQ allows it to carry out thou- sands of electron transfers without undergoing molecular breakdown. It has been proven especially effective in neutralizing the ubiquitous superoxide and hydroxyl radicals. [36] According to the most recent research, “PQQ is 30 to 5,000 times more efficient in sustaining redox cycling . . . than other common [antioxidant compounds], e.g. ascorbic acid.” [37]
Protection Against Brain Aging
PQQ has been shown to optimize function of the entire central nervous system. It reverses cognitive impairment caused by chronic oxidative stress in pre-clinical models, improving performance on memory tests. [40] It has also been shown to safeguard a gene involved in the development of Parkinson’s disease (called DJ-1) from self-oxidation—an early step in the onset of Parkinson’s. [41]
Reactive nitrogen species (RNS), like reactive oxygen species, impose severe stresses on damaged neurons. [42] They arise spontaneously following stroke and spinal cord injuries, and have been shown to account for a substantial proportion of subsequent long- term neurological damage. PQQ directly suppresses RNS in experimentally induced strokes. [43] It also provides additional protection by blocking gene expression of inducible nitric oxide synthase, a major source of RNS, following spinal cord injury. [44]
PQQ protects brain cells against damage following ischemia-reperfusion injury— the inflammation and oxidative damage that result from the sudden return of blood and nutrients tissues deprived of them by stroke. [45] Given immediately before induction of stroke in animal models, PQQ significantly reduces the size of the damaged brain area. [46] This finding implies that if a person were to suffer a temporary loss of cerebral blood flow due to cardiac arrest, stroke, or trauma, that having PQQ in their body would afford considerable protection against permanent brain damage.
PQQ also beneficially interacts with brain neurotransmitter systems. In particular, PQQ protects neurons by modifying the important NMDA receptor site. [47,48] NMDA is a powerful mediator of “excitotoxicity,” a response to long-term overstimulation of neurons that is associated with many neurodegenerative diseases and seizures. [49-51] PQQ protects against neurotoxicity induced by other toxins, including mercury. [52,53]
A mounting body of evidence points to PQQ as a potent intervention in Alzheimer’s and Parkinson’s disease. Both are triggered by accumulation of abnormal proteins that initiate a cascade of oxidative events resulting in brain cell death. PQQ prevents development of alpha-synuclein, the protein responsible for Parkinson’s disease. [54] It also protects nerve cells from the oxidizing ravages of the Alzheimer’s- causing amyloid-beta protein. [55] A 2010 study revealed that PQQ could prevent formation of amyloid-beta molecular structures. [56] These effects were traced to three distinct biochemical mechanisms described earlier.
PQQ has also been shown to protect memory and cognition in aging animals and humans. [22,57] It stimulates production and release of nerve growth factor in cells that support neurons in the brain. [58] This may partially explain why PQQ supplementation of aging rats resulted in marked retention of their maximum memory function. [57] In humans, supplementation with 20 mg per day of PQQ resulted in improvements on tests of higher cognitive function in a group of middle-aged and elderly people. [22]
PQQ has also been shown to protect memory and cognition in both aging animals and humans.
These effects were significantly amplified when the subjects also took 300 mg per day of CoQ10. Presumably a lower dose of the more absorbable ubiquinol form of CoQ10 would provide the same benefit as 300 mg of ubiquinone.
Cardiovascular Defense
As with strokes, damage in heart attacks is inflicted via ischemia-reperfusion injury. Ischemia-reperfusion means loss of blood flow (ischemia) to part of the body and the subsequent re-flow (reperfusion) when blood flow is restored. Cells are injured when blood flow is interrupted and often sustain even greater damage when blood flow is suddenly restored. Supplementation with PQQ reduces the size of ischemia-reperfusion damaged areas in animal models of acute myocardial infarction (heart attack). [5] This occurs whether the supplement is given before or after the ischemic event itself.
To further investigate this potential, researchers at the VA Medical Center at UC-San Francisco compared PQQ with metoprolol, a commonly prescribed beta blocker that is standard post-heart attack clinical treatment. [60] Given alone, both treatments reduced the damaged areas’ size and protected against heart muscle dysfunction. When they were given together, the left ventricle’s pumping pressure was enhanced. The combination also increased mitochondrial energy-producing functions—but the effect was small compared with the better response seen with PQQ alone. [60] And only PQQ favorably reduced lipid peroxidation. The remarkable conclusion: “PQQ is superior to metoprolol in protecting mitochondria from ischemia/reperfusion oxidative damage.” [60]
Subsequent research from the same team has demonstrated that PQQ helps heart muscle cells resist acute oxidative stress. [61] The mechanism? Preserving and enhancing mitochondrial function.
Why Mitochondria are so Vulnerable to Free Radical damage
The death spiral of our mitochondria is accelerated by the very physiological function they must perform, i.e. energy production. As the cell’s power generators, mitochondria are the site of enormous and constant oxidative activity that spews out toxic free radicals. To make matters worse, relative to nuclear DNA, mitochondrial DNA possesses few defenses against free radical damage. [38,39]
DNA in the cell’s nucleus is protected by numerous “guardian” proteins that blunt the impact of free radicals. No such repair systems exist to protect mitochondrial DNA.
Nuclear DNA also enjoys superior structural defenses. It is housed within a protective double-membrane that separates it from the rest of the cell. This double-membrane is complemented by a dense matrix of filament proteins called the nuclear lamina, a kind of hard shell casing to further buffer DNA from external impacts.
By comparison, mitochondrial DNA is left almost entirely exposed: it attaches directly to the inner membrane where the mitochondria’s electrochemical furnace rages continuously, generating an enormous volume of toxic reactive oxygen species. This is why supplementation with lipoic acid, carnosine, and other mitochondrial-protecting antioxidants is so important.
The extraordinary antioxidant capacity of PQQ represents a powerful new intervention that may effectively reinforce the mitochondria’s meager defenses.
Summary
Cellular aging is intimately associated with the decline in mitochondrial number and functionality. Nutrients that provide pro- tection to existing mitochondria include resveratrol, carnosine, lipoic acid, L-carnitine, and CoQ10. During the course of normal aging, however, the number of functional mitochondria pathologically diminishes, leading to a host of debilitating disorders followed by death of the organism. For the first time in scientific history, a natural compound called PQQ is available to increase the functionality of existing mitochondria while promoting the generation of new mitochondria inside aging cells.
This article is copyright 2010 by Life Extension Magazine (R), a sponsor of H+ Magazine, and is reprinted with permission.
To order PQQ, please call Life Extension at 1-866-748-7538, or visit www.lef.org/hplus.
References
1. Bliznakov EG. Aging, mitochondria, and coenzyme Q(10): the neglected relationship. Biochimie. 1999 Dec;81(12):1131-2.
2. Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989 Mar 25;1(8639):642-5
3. Lanza IR, Nair KS. Mitochondrial metabolic function assessed in vivo and in vitro. Curr Opin Clin Nutr Metab Care. 2010 Jul 7.
4. Mota MP, Peixoto FM, Soares JF, et al. Influence of aerobic fitness on age-related lymphocyte DNA damage in humans: relationship with mitochondria respiratory chain and hydrogen peroxide production. Age (Dordr). 2010 Mar 20.
5. Tranah G. Mitochondrial-nuclear epistasis: Implications for human aging and longevity. Ageing Res Rev. 2010 Jun 25.
6. Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci. 2010 Jun 25.
7. Wei YH, Ma YS, Lee HC, Lee CF, Lu CY. Mitochondrial theory of aging matures—roles of mtDNA mutation and oxidative stress in human aging. Zhonghua Yi Xue Za Zhi (Taipei). 2001 May;64(5):259-70.
8. Linnane AW, Kovalenko S, Gingold EB. The universality of bioenergetic disease: age-associated cellular bioenergetic degradation and amelioration therapy. Ann N Y Acad Sci. 1998 Nov 20;854:202-13.
9. Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010 Jul 16.
10. Conley KE, Amara CE, Jubrias SA, Marcinek DJ. Mitochondrial function, fibre types and ageing: new insights from human muscle in vivo. Exp Physiol. 2007 Mar;92(2):333-9.
11. Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia—reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001 Jun;33(6):1065-89.
12. Maruszak A, Zekanowski C. Mitochondrial dysfunction and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010 Jul 15.
13. Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann N Y Acad Sci. 2006 May;1067:182-90.
14. Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1 alpha expression. J Biol Chem. 2010 Jan 1;285:142-52.
15. Bruce A, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York, NY: Garland Publishing, Inc.;1994.
16. Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry: Life at the Molecular Level. 2nd ed. New Jersey: John Wiley and Sons, Inc.; 2006:547.
17. Pike RL, Brown M. Nutrition: An Integrated Approach. New York, NY: Prentice-Hall; 1984:450-84.
18. Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010 Jan;459(2):277-89.
19. Robb EL, Page MM, Stuart JA. Mitochondria, cellular stress resistance, somatic cell depletion, and life span. Curr Aging Sci. 2009 Mar;2(1):12-27.
20. Alexeyev MF, LeDoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci. 2004;107:355-364.
21. Rucker R, Chowanadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Altern Med Rev. 2009 Sep;14(3):268-77.
22. Nakano M, Ubukata K, Yamamoto T, Yamaguchi H. Effect of pyrroloquinoline quinone (PQQ) on mental status of middle-aged and elderly persons. FOOD Style. 2009;21:13(7):50-3.
23. Smidt CR, Bean-Knudsen D, Kirsch DG, Rucker RB. Does the intestinal microflora synthesize pyrroloquinoline quinone? Biofactors.1991 Jan;3(1):53-9.
24. Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000 Apr;130(4):719-27.
25. Choi O, Kim J, Kim JG, et al. Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol. 2008 Feb;146(2):657-68.
26. Stites T, Storms D, Bauerly K, et al. Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J Nutr. 2006 Feb;136(2):390-6.
27. Steinberg F, Stites TE, Anderson P, et al. Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp Biol Med (Maywood). 2003 Feb;228(2):160-6.
28. Bauerly KA, Storms DH, Harris CB, et al. Pyrroloquinoline quinone nutritional status alters lysine metabolism and modulates mitochondrial DNA content in the mouse and rat. Biochim Biophys Acta. 2006 Nov;1760(11):1741-8.
29. Entrez Gene: PPARGC1A peroxisome proliferator- activated receptor gamma, coactivator 1 alpha [ Homo sapiens ] GeneID: 10891.
30. Entrez Gene: CREBBP CREB binding protein [ Homo sapiens ] GeneID: 1387.
31. Zhong N, Xu J. Synergistic activation of the human MnSOD promoter by DJ-1 and PGC-1alpha: regulation by SUMOylation and oxidation. Hum Mol Genet. 2008 Nov 1;17(21):3357-67.
32. Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Rad Res. 2001; 35(6):885-93.
33. Nunome K, Miyazaki S, Nakano M, Iguchi-Ariga S, Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol Pharm Bull. 2008 Jul;31(7):1321-6.
34. Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004 Feb;5(2):213-8.
35. Paz MA, Martin P, Fluckiger R, Mah J, Gallop PM. The catalysis of redox cycling by pyrroloquinoline quinone (PQQ), PQQ derivatives, and isomers and the specificity of inhibitors. Anal Biochem. 1996;238:145-9.
36. Urakami T, Yoshida C, Akaike T, Maeda H, Nishigori H, Niki E. Synthesis of monoesters of pyrroloquinoline quinone and imidazopyrroloquinoline, and radical scavenging activities using electron spin resonance in vitro and pharmacological activity in vivo. J Nutr Sci Vitaminol (Tokyo). 1997 Feb;43(1):19-33.
37. Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000 Apr;130(4):719-27.
38. Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27:647-53.
39. Miquel J. An update on the mitochondrial-DNA mutation hypothesis of cell aging. Mutat Res. 1992 Sep;275(3-6):209-16.
40. Ohwada K, Takeda H, Yamazaki M, et al. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J Clin Biochem Nutr. 2008 Jan;42:29-34.
41. Nunome K, Miyazaki S, Nakano M, Iguchi-Ariga S, Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol Pharm Bull. 2008 Jul;31(7):1321-6.
42. Ono K, Suzuki H, Sawada M. Delayed neural damage is induced by iNOS-expressing microglia in a brain injury model. Neurosci Lett. 2010 Apr 5;473(2):146-50.
43. Zhang Y, Rosenberg PA. The essential nutrient pyrroloquinoline quinone may act as a neuroprotectant by suppressing peroxynitrite formation. Eur J Neurosci. 2002 Sep;16(6):1015-24.
44. Hirakawa A, Shimizu K, Fukumitsu H, Furukawa S. Pyrroloquinoline quinone attenuates iNOS gene expression in the injured spinal cord. Biochem Biophys Res Commun. 2009 Jan 9;378(2):308-12.
45. Jensen FE, Gardner GJ, Williams AP, Gallop PM, Aizenman E, Rosenberg PA. The putative essential nutrient pyrroloquinoline quinone is neuroprotective in a rodent model of hypoxic/ischemic brain injury. Neuroscience. 1994 Sep;62(2):399-406.
46. Zhang Y, Feustel PJ, Kimelberg HK. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006 Jun 13;1094(1):200-6.
47. Aizenman E, Hartnett KA, Zhong C, Gallop PM, Rosenberg PA. Interaction of the putative essential nutrient pyrroloquinoline quinone with the N-methyl- D-aspartate receptor redox modulatory site. J Neurosci. 1992 Jun;12(6):2362-9.
48. Aizenman E, Jensen FE, Gallop PM, Rosenberg PA, Tang LH. Further evidence that pyrroloquinoline quinone interacts with the N-methyl-D-aspartate receptor redox site in rat cortical neurons in vitro. Neurosci Lett. 1994 Feb 28;168(1-2):189-92.
49. Hossain MA. Molecular mediators of hypoxic-ischemic injury and implications for epilepsy in the developing brain. Epilepsy Behav. 2005 Sep;7(2):204-13.
50. Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009 Apr;30(4):379-87.
51. Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009 Jul;11(7):1587-602.
52. Hara H, Hiramatsu H, Adachi T. Pyrroloquinoline quinone is a potent neuroprotective nutrient against 6-hydroxydopamine-induced neurotoxicity. Neurochem Res. 2007 Mar;32(3):489-95.
53. Zhang P, Xu Y, Sun J, Li X, Wang L, Jin L. Protection of pyrroloquinoline quinone against methylmercury- induced neurotoxicity via reducing oxidative stress. Free Radic Res. 2009 Mar;43(3):224-33.
54. Kobayashi M, Kim J, Kobayashi N, et al. Pyrroloquinoline quinone (PQQ) prevents fibril formation of alpha-synuclein. Biochem Biophys Res Commun. 2006 Oct 27;349(3):1139-44.
55. Zhang JJ, Zhang RF, Meng XK. Protective effect of pyrroloquinoline quinone against Abeta-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 2009 Oct 30;464(3):165-9.
56. Kim J, Kobayashi M, Fukuda M, et al. Pyrroloquinoline quinone inhibits the fibrillation of amyloid proteins. Prion. 2010 Jan;4(1):26-31.
57. Takatsu H, Owada K, Abe K, Nakano M, Urano S. Effect of vitamin E on learning and memory deficit in aged rats. J Nutr Sci Vitaminol (Tokyo). 2009;55(5):389-93.
58. Murase K, Hattori A, Kohno M, Hayashi K. Stimulation of nerve growth factor synthesis/secretion in mouse astroglial cells by coenzymes. Biochem Mol Biol Int. 1993 Jul;30(4):615-21.
59. Zhu BQ, Zhou HZ, Teerlink JR, Karliner JS. Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size and improves cardiac function in rat models of ischemia and ischemia/reperfusion. Cardiovasc Drugs Ther. 2004 Nov;18(6):421-31.
60. Zhu BQ, Simonis U, Cecchini G, et al. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther. 2006 Jun;11(2):119-28.
61. Tao R, Karliner JS, Simonis U, et al. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem Biophys Res Commun. 2007 Nov 16;363(2):257-62.
Special Forces Training for Weight Vest Enthusiasts
I came across a couple of decent special forces selection preparation programs yesterday that are ideal for weight vest owners. If you want to maximise your weight vest training this is the way to go. Just use your vest instead of the pack.
Its better for your body with a vest. Before I post the programs here are some videos to whet your appetite. The first is on Australian SAS selection which looks bloody tough. This is a full documentary – its good too.
The second is on New Zealand SAS whom I was fortunate enough to do some training with many years ago as an infantryman in New Zealands Army.
There are 18 parts to that one so search the rest on youtube. Just remember when the guys do it in the Army its without food or sleep unlike civilians who usually are not that intense. Also with the military approach you will inevitably be injured and often quite seriously. I don’t encourage you to push that hard as a civilian athlete.
The Australians have a very intense selection process including an initial 20km ruck march to be completed in 3 hours 15 minutes with a 20 kg pack, an insane exercise called “happy wanderer” carrying over 100lbs 150 km over a mountain range for 5 days alone. That’s over rocks and up very steep terrain & in the middle of a bunch of other insanely difficult testing. They also do a six hour 10 km march with a full pack and two 20 liter jerry cans, That also is a monolithic killer.
The New Zealand SAS has an exercise called Von Temsky which involves a 77lb pack, final march is (60km) 37miles, pace is relatively slow, 3km per hour. Operation Von Tempsky is a ball breaker, 24hrs marching with ruck, webbing rifle through a swamp while carrying alternately one or two full 20L jerry cans.
Swamp VS mountain ? yeah what ever
OK so that basically is coming close to the limits of human endurance so You know how high you can strive – remember they do it without food or sleep too. On operations they do worse stuff.
I was going to post these here but its a hassle – instead you can download them:
http://www.freefitnessguru.com/Downloads/13weekSFtrainingprogramme.doc
http://www.freefitnessguru.com/Downloads/6WEEKTRAININGPROGRAM.doc
http://www.freefitnessguru.com/Downloads/SAS selection 13 week prep.pdf
Bison 1&2 Freaky Cool Russian Grip Gear
Yesterday was xmas day & I was writing the sales copy for my new book at www.GripTrainingHandbook.com when I came across some Russian equipment I was previously unfamiliar with. Its called the Bison-1.




Now immediately upon sight I know this is something I want and will buy. Here are some Youtube vids too…
OK if that was not freaky enough they have a new device called the Bison-2

Yeah i want one of these too. I looked around for suppliers as I beleive the Bison-1 is around US$150.00 from the manufacturer. http://beinsport.com/ is offering either device for under US$100.00 each free shipping but I can not vouch for the site.
Looks very cool to me.
Cardio indoors with zero equipment
I just did a full days work – not hard, I was painting a house. I got home tired and crashed out for 3 hours when I awoke it was 9:30pm and I felt like shit. Like I really needed a workout to burn some fat.
Outside its wet, raining. So I had all of these excuses in my head, rain, late in evening, I’m tired etc.
Solution: 30 minutes running on the spot, star jumps, skipping without a rope and jumping in place.
The result: I am sweating like a pig, my heart has been racing for 30 minutes & my calf muscles are swollen with blood and lactic acid.
Equipment required: 1 square meter of ground, one towel and one bottle of water.
No excuses & no reason this cant be done 3x per day or more.
My new book – The Grip training Handbook
I have a new book out which is just about to launch “The Grip Training Handbook”
www.GripTrainingHandbook.com
Its chock full of tips , exercises and routines for getting super hands and fore arms

This is a heavily illustrated manual that will get you strong hands if you take advantage of the advice. Hand strength comes quickly as the muscles are only small but most people neglect this aspect of the strength chain.
As bonus’s I will include an upgraded version on my HGH guide, testoserone guide and Rib Cage expansion guide.
Once this book is launched I will follow up with a Kettlebell manual and a weight vest training manual.
You can get my “free” reports from site:freefitnessguru.com/Downloads/
These will all be upgraded before being offered as bonus reports. The grip Training manual will be priced at US $37.97
My other book is “Iron Power Palm” on kung fu breaking conditioning – yes I can smash concrete with my hands.

Notorious Liverpool street – a mean hill
I found a mean as hell hill 2 minutes walk from where I live. Its on Liverpool street and I swear this mother is on a real steep incline. I can only do about 6-7 times up it in 40 minutes without puking or hyper ventilating.
I am talking about wearing a 40lb weight vest at the same time of course.
Soon I’ll get a tire or two and some cables and do a hand pull/push sled with weight vest. Then I may well pass out or collapse almost.
Anyway this video doesn’t do justice to the hill & makes it look short and easy but its actually a mean hill.
This dude skating used to live in my neighborhood Herne Bay 20 years ago but I don’t know him. He’s a bit of a local character. I just wanted to show you guys the hill.
16 year old athlete training
OK I just saw this story in the New Zealand herald and had to share it with you guys.
This kid is only 16 and I did have a few buddies as big and strong as him at sixteen but they were forestry workers. Some of the training this guy does he definitely wont be doing five years from now. I think you can guess why.
None the less for sixteen he is very big and pretty strong.
Heres the video & story from the nzherald…
If the fact he’s reached the Olympic shot put qualifying mark at aged 16 doesn’t impress you then maybe Jacko Gill’s training methods will.
A six minute training video posted on YouTube showcases the impressive strength that Gill, one of the country’s most promising athletes in recent history, has built up as he eyes a potential medal at next year’s Olympics in London.
Some of his training methods are unusual, like jumping over a fellow teen and one-arm bench pressing a dog, but it also features some impressive work on a punching bag and medicine ball for someone his age.
In the video he also dunks a basketball hoop with a shot put, bench presses big weights with relative ease and jumps onto a 1.55m high platform from a starting start.
His father Walter told the Herald on Sunday that Jacko, who quit school to concentrate fulltime on his shot put career, trains all through the night.
“When he goes into a build-up phase, he does three training sessions a day,” says dad Walter.
“Some of them are in the middle of the night. The house shakes and the dog runs outside. We might be asleep, and he’ll be lifting weights or bounding up and down the stairs to our bedroom door.
“He’s definitely different. How we ever produced that, I’ll never know, but we’re very proud of him.”
Tweaking a gene makes muscles twice as strong
A team of researchers at EPFL, the University of Lausanne and the Salk Institute created super strong, marathon mice and nematodes by reducing the function of a natural inhibitor, suggesting treatments for age-related or genetically caused muscle degeneration are within reach.
It turns out that a tiny inhibitor may be responsible for how strong and powerful our muscles can be. This is the surprising conclusion reached by scientists in EPFL’s Laboratory of Integrative Systems Physiology (LISP), in collaboration with a group in the Center for Integrative Genomics at the University of Lausanne and at the Salk Institute in California. By acting on a receptor (NCoR1), they were able to modulate the transcription of certain genes, creating a strain of mighty mice whose muscles were twice a strong as those of normal mice.
Two protein-building regulators
The process of transcription, in which proteins are manufactured by an organism in response to instructions contained in its DNA, is modulated by co-factors. These either favor (coactivators) or inhibit (corepressors) transcription, in principle by responding to the concentration of certain hormones in the body, which are in turn associated with the organism’s environment.
In an article appearing today in the journal Cell, a team led by EPFL professor Johan Auwerx reports on the results of experiments done in parallel on mice and nematodes. By genetically manipulating the offspring of these species, the researchers were able to suppress the NCoR1 corepressor, which normally acts to inhibit the buildup of muscle tissues.
Better muscles
In the absence of the inhibitor, the muscle tissue developed much more effectively. The mice with the mutation became true marathoners, capable of running faster and longer before showing any signs of fatigue. In fact, they were able to cover almost twice the distance run by mice that hadn’t received the treatment. They also exhibited better cold tolerance.
Unlike previous experiments with so-called super mice, this study addresses the way energy is burned in the muscle and the way the muscle is built. Examination under a microscope confirmed that the muscle fibers of the modified mice are denser, the muscles are more massive, and the cells in the tissue contain higher numbers of mitochondria–cellular organelles that deliver energy to the muscles.
Similar results were also observed in nematode worms, allowing the scientists to conclude that their results could be applicable to a large range of living creatures.
Obese but not diabetic
According to a second article published in the same journal and also involving EPFL’s LISP Laboratory, suppressing the NCoR1 receptor in adipose tissues (fat) also led to interesting results. By acting on this corepressor, it was possible to fundamentally change the corpulence of the mice being studied without inducing weight-related diseases. “The specimens that became obese via this treatment did not suffer from diabetes, unlike mice who become obese for other reasons,” notes Auwerx.
The scientists have not yet detected any deleterious side effects associated with eliminating the NCoR1 receptor from muscle and fat tissues, and although the experiments involved genetic manipulations, the researchers are already investigating potential drug molecules that could be used to reduce the receptor’s effectiveness.
Treating degeneration
The researchers say their results are a milestone in our understanding of certain fundamental mechanisms of living organisms, in particular the little-studied role of corepressors. In addition, they give a glimpse at possible long-term therapeutic applications. “This could be used to combat muscle weakness in the elderly, which leads to falls and contributes to hospitalizations,” emphasizes Auwerx. “In addition, we think that this could be used as a basis for developing a treatment for genetic muscular dystrophy.”
If these results are confirmed in humans, there’s no question it will attract interest from athletes as well as medical experts. “It will be important for anti-doping authorities to monitor that these treatments are not used in an unauthorized manner,” concludes Auwerx.